Millikan Oil Drop Experiment
Discover how Robert Millikan determined the fundamental charge of an electron through his ingenious oil drop experiment. Use our interactive simulation to recreate this Nobel Prize-winning experiment.
Key Learning Outcomes:
- • Understand the experimental setup and methodology
- • Measure terminal velocities of charged oil droplets
- • Calculate the charge on individual droplets
- • Discover the quantized nature of electric charge
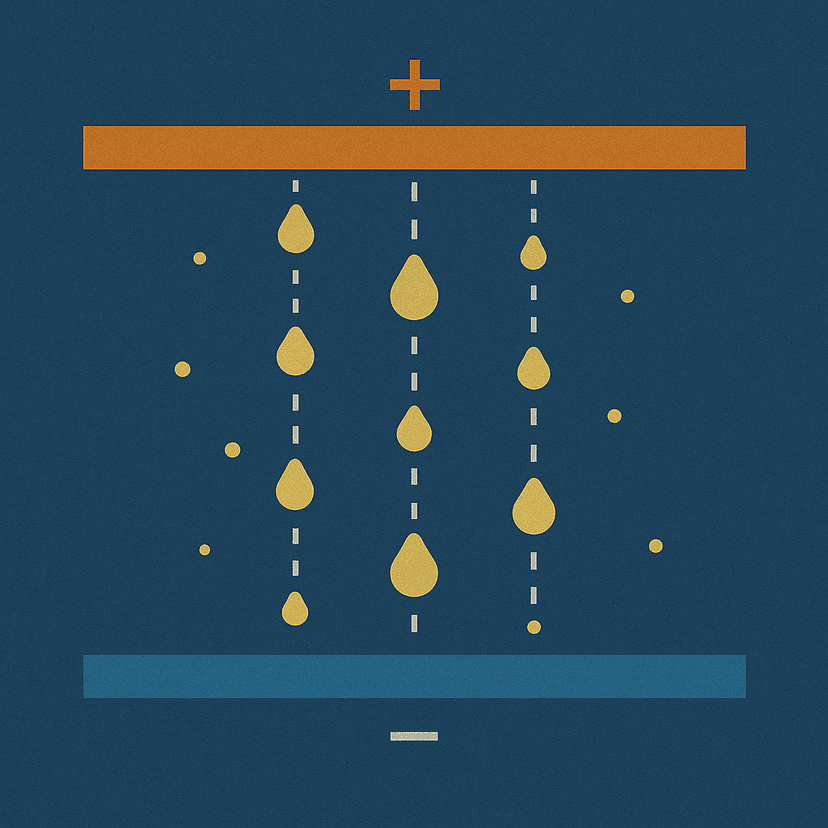
Interactive Millikan Oil Drop Experiment
Instructions:
• Spray oil droplets to create the tracking droplet
• Watch it fall along the crosshair line
Current Phase:
Setup - Spray droplets
Measurement:
Crosshairs at 3mm and 13mm from top plate
Distance: 10.0mm exactly
No measurements yet.
Start by spraying droplets!
🔬 Precise Measurement
Crosshairs positioned exactly 10mm apart within the chamber for accurate velocity measurements using realistic physics.
📍 Tracking Droplet
Automatically follows the vertical measurement line between crosshairs for consistent and accurate results.
🧮 Reverse Calculation
Droplet radius and charge are calculated from measured velocities, exactly like in Millikan's original experiment.
Historical Context
While Thomson's experiment proved that negatively charged particles existed and provided their charge-to-mass ratio, the actual values remained unknown. Millikan's oil drop experiment determined these fundamental constants.
Experimental Setup
The experimental setup consisted of:
- • Two metal plates connected to a DC voltage source
- • A small gap of 16 mm between the plates
- • An atomizer to spray oil droplets
- • X-ray source to ionize air molecules
- • A microscope with crosshairs and vertical scale
Methodology
Step 1: Oil droplets were sprayed while voltage was off. Air was ionized with X-rays, causing electrons to attach to droplets.
Step 2: Terminal velocity v₁ was measured as droplets fell under gravity (voltage off). Time was recorded for a fixed distance.
Step 3: Voltage was applied upward, causing charged droplets to rise. Terminal velocity v₂ was measured.
Step 4: Using both velocities, the charge on individual droplets was calculated using force balance equations.
Key Discovery
Millikan found that charges were always integer multiples of a fundamental value: 1.6 × 10⁻¹⁹ C. This proved that electric charge is quantized and determined the elementary charge of an electron.
Step 1: Force Analysis (Voltage OFF)
When the voltage is off, the oil droplet falls at terminal velocity v₁. The forces are balanced:
Using Stokes' law and Archimedes' principle:
Solving for radius r:
Step 2: Force Analysis (Voltage ON)
When voltage V is applied, the droplet rises at terminal velocity v₂. The electric field creates an upward force:
Step 3: Final Formula
Solving for the charge q:
This formula allows us to calculate the charge on an oil droplet from the measured terminal velocities and known constants.
Key Result:
Millikan found that all measured charges were integer multiples of:
This proved that electric charge is quantized.