Discovery of the Electron
Experience JJ Thomson's groundbreaking cathode ray experiment that led to the discovery of the electron and revolutionized our understanding of atomic structure.
The Cathode Ray Mystery (1850s-1890s)
Since the mid-1800s, scientists observed mysterious glowing phenomena in evacuated glass tubes. Two competing theories emerged: William Crookes proposed his "radiant matter theory" suggesting cathode rays were electrically charged atoms, while Heinrich Hertz and Eugen Goldsteinbelieved they were aether vibrations (electromagnetic waves). Thomson's 1897 experiment finally resolved this debate by proving cathode rays were streams of charged particles.
What we'll Learn
- • How electric and magnetic fields deflect cathode rays
- • How Thomson calculated the charge-to-mass ratio (e/m)
- • Why this proved cathode rays are particles, not waves
- • How this discovery led to the electron and atomic structure
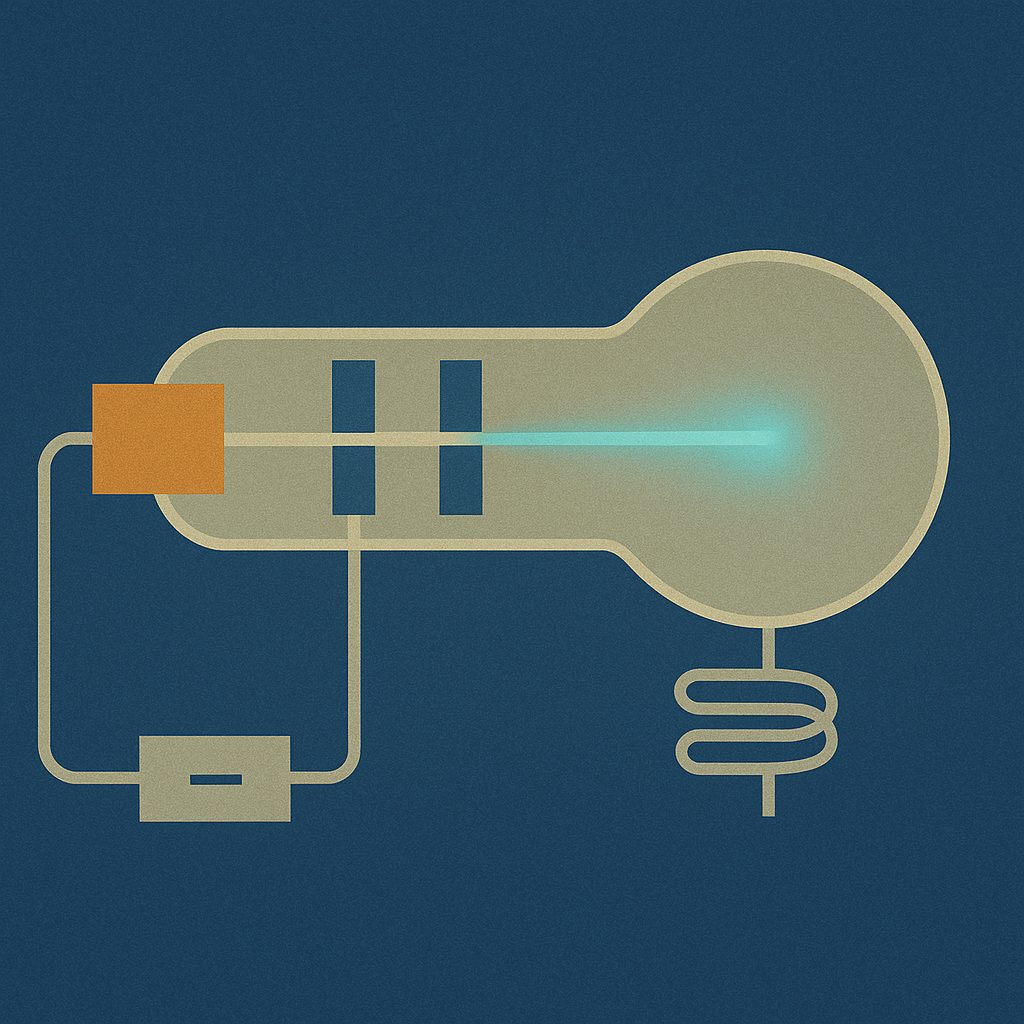
Stage 1: Cathode Ray Tube Setup
Turn on the power and adjust the vacuum level to see a clear electron beam. Notice how the beam travels from cathode to anode and hits the phosphorescent screen.
Basic Controls
Vacuum System
Higher vacuum = clearer beam path
Quick Actions
What happens to the cathode ray beam when you increase the vacuum level?