Atomic Physics
Discover how scientists broke open the atom and found a whole world inside
Ancient Greek and Indian philosophers proposed that everything was made of atoms. Dalton's atomic theory gave this idea scientific foundation. But what were atoms themselves made of? Discover the step-by-step journey of how scientists unraveled the mysteries of atomic structure through the discovery of subatomic particles and revolutionary experiments.
Interactive Atomic Physics Concepts
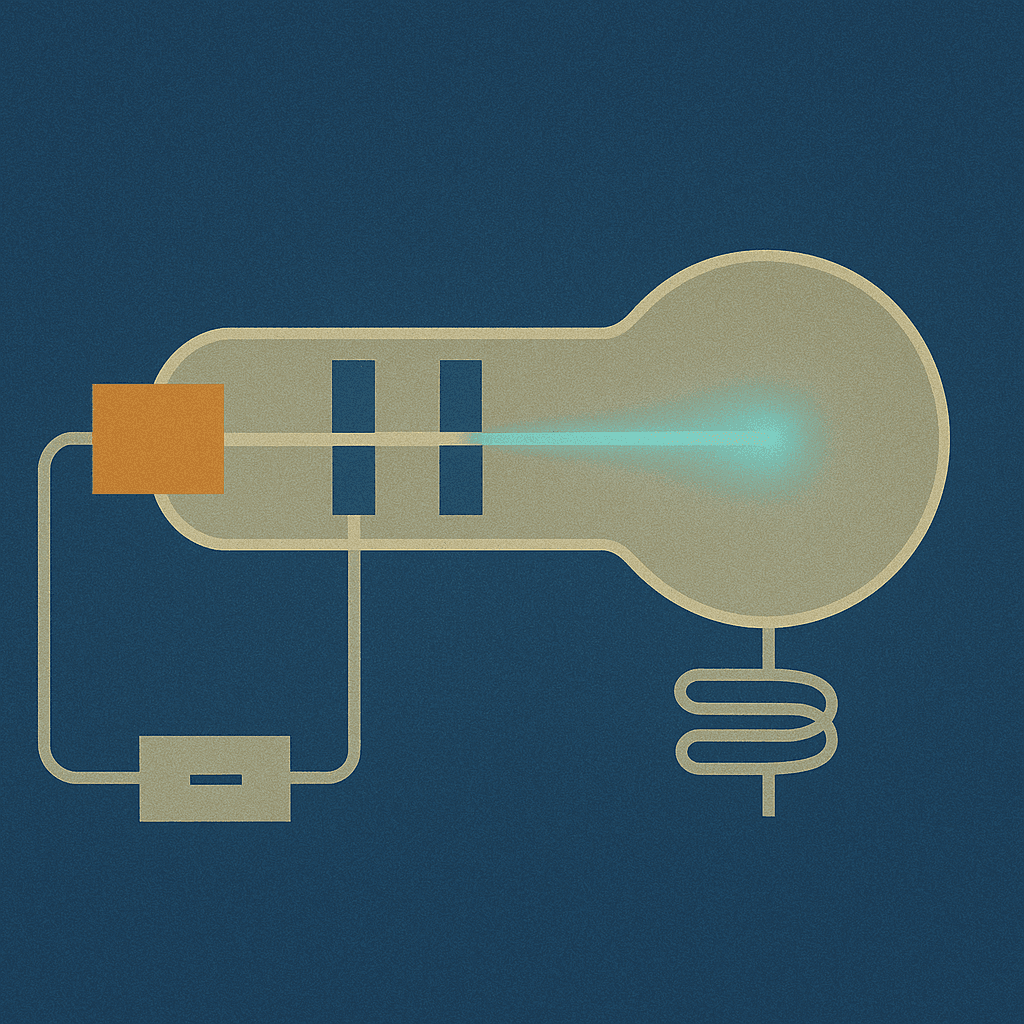
JJ Thomson's Experiment
Discovery of the electron and its charge/mass ratio
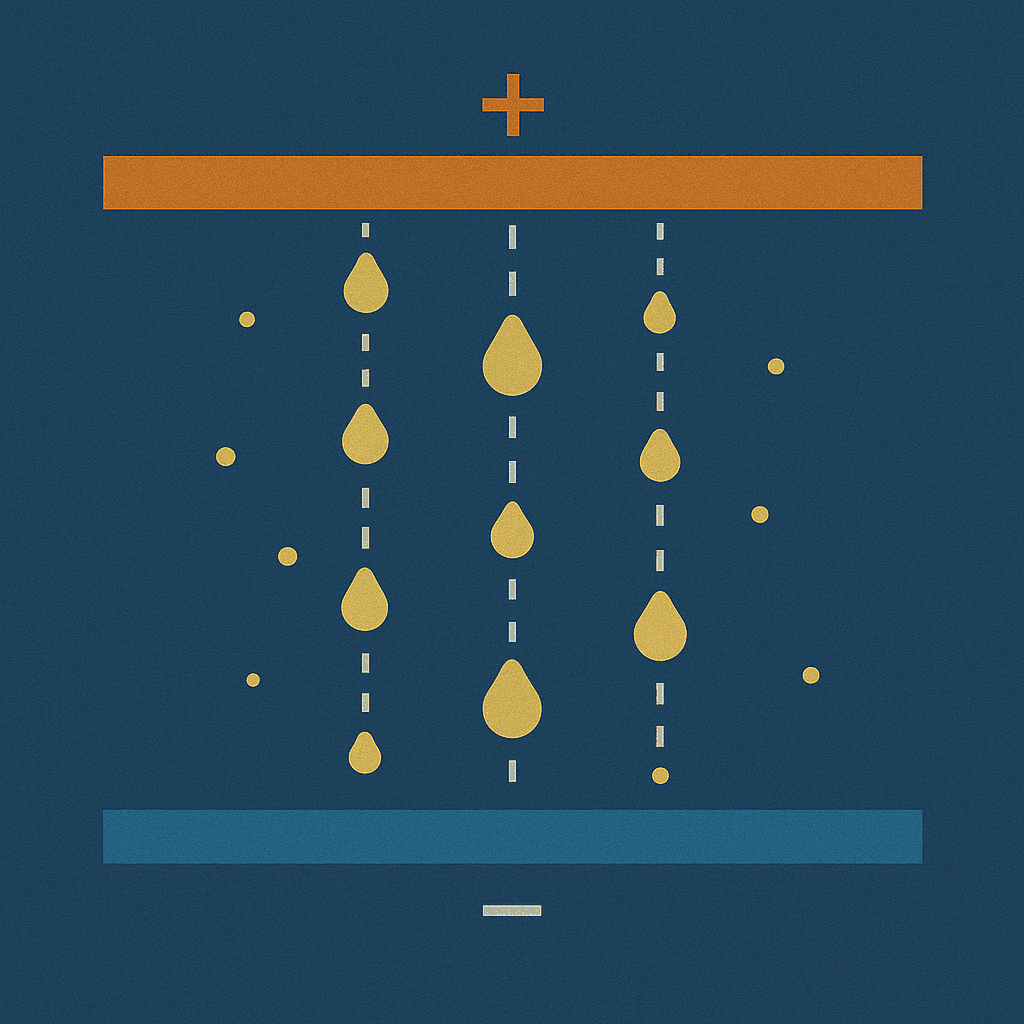
Millikan Oil Drop
Measuring the charge and mass of a single electron
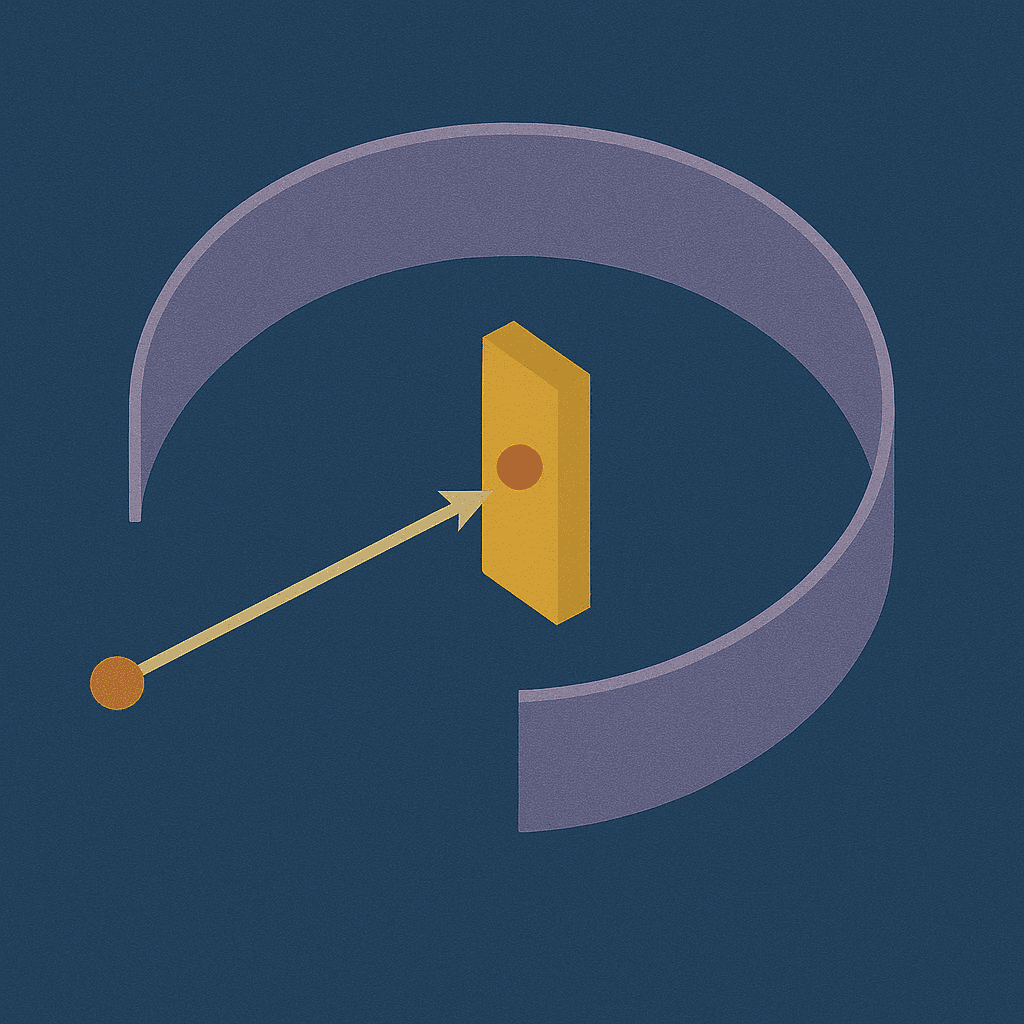
Rutherford Scattering
Unveiling the atomic nucleus with alpha particles
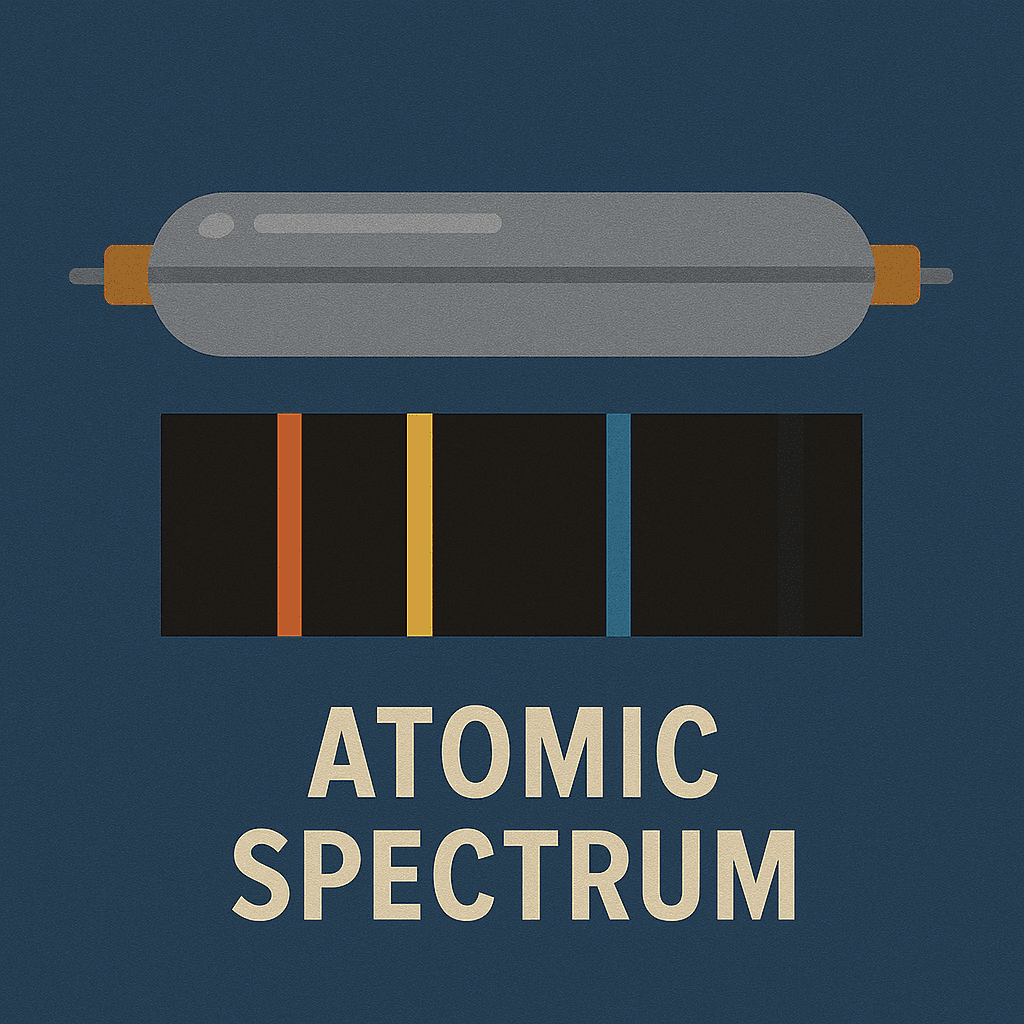
Hydrogen Spectrum
The mysterious colored lines of hydrogen

Bohr Model
Electrons in orbits, quantized energy levels

De Broglie Hypothesis
Electron as a standing wave that closes on itself
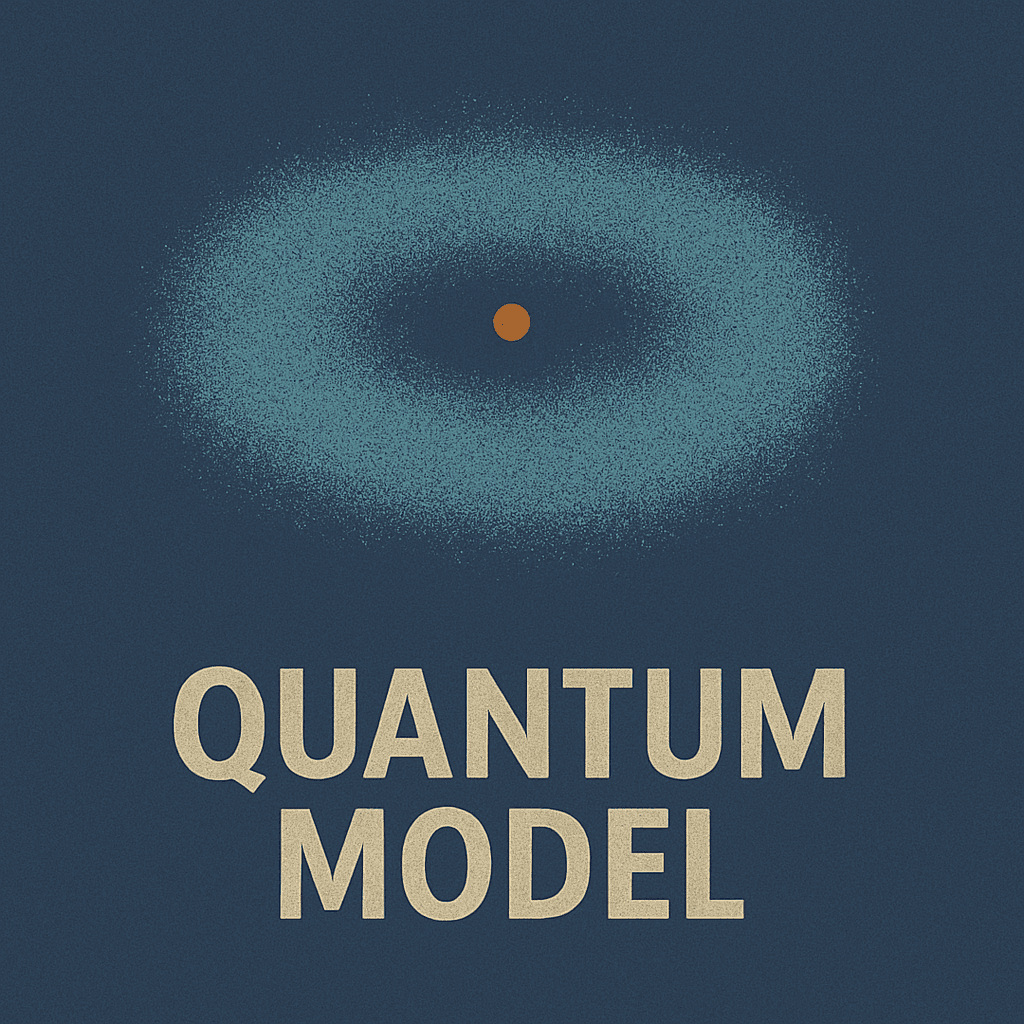
Quantum Model
Electron clouds and probabilistic orbitals
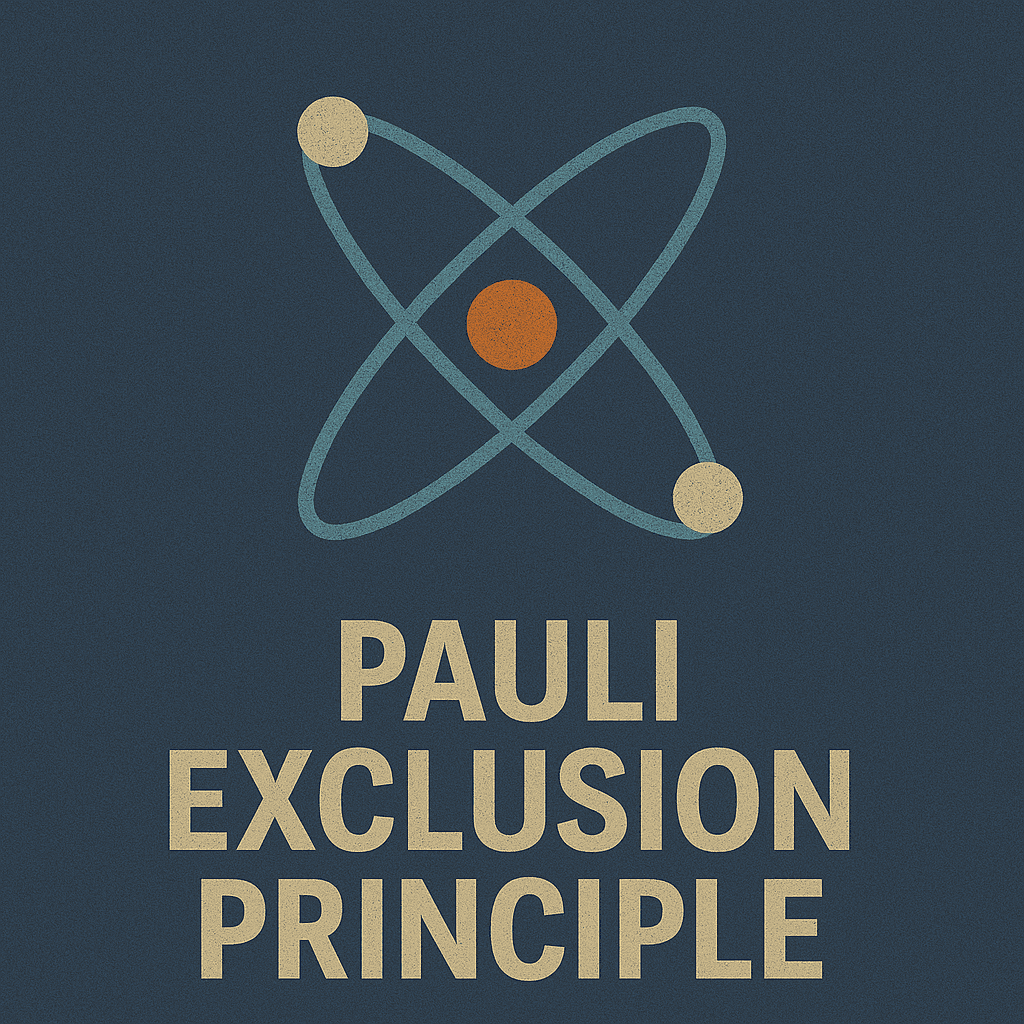
Pauli Exclusion Principle
No two electrons can occupy the same quantum state
How These Concepts Connect
This is not just a collection of separate discoveries — it's a unified story of how scientists built upon each other's work to understand atomic structure.
Electron Discovery
Thomson's cathode ray experiments revealed charged particles
Electron Properties
Millikan measured precise charge and mass values
Nuclear Atom
Rutherford discovered the dense atomic nucleus
Atomic Spectrum
Hydrogen's discrete spectral lines revealed energy levels
Bohr Model
Quantized orbits explained spectral observations
Wave-Particle Duality
De Broglie showed electrons have wave properties
Quantum Model
Probability clouds replaced definite orbits
Pauli Principle
No two electrons share identical quantum states
The Complete Journey
From the discovery of subatomic particles to the modern quantum mechanical model, each experiment and theory built upon previous knowledge to reveal the true nature of atoms.
Suggested Learning Path
Want to follow the historical journey step by step? Start here and discover atomic structure in the same order scientists did.